Allogeneic Cell Therapy Devices Market By Therapy Type (Stem Cell Therapies, Non-stem Cell Therapies), By Therapeutic Area (Hematological Disorders, Dermatological Disorders, Others), By End-User (Hospitals & Clinics, Research Institutes & Academic Centers, Biotechnology & Pharmaceutical Companies, Others), Region and Companies – Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2024-2033
- Published date: March 2024
- Report ID: 117463
- Number of Pages: 265
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
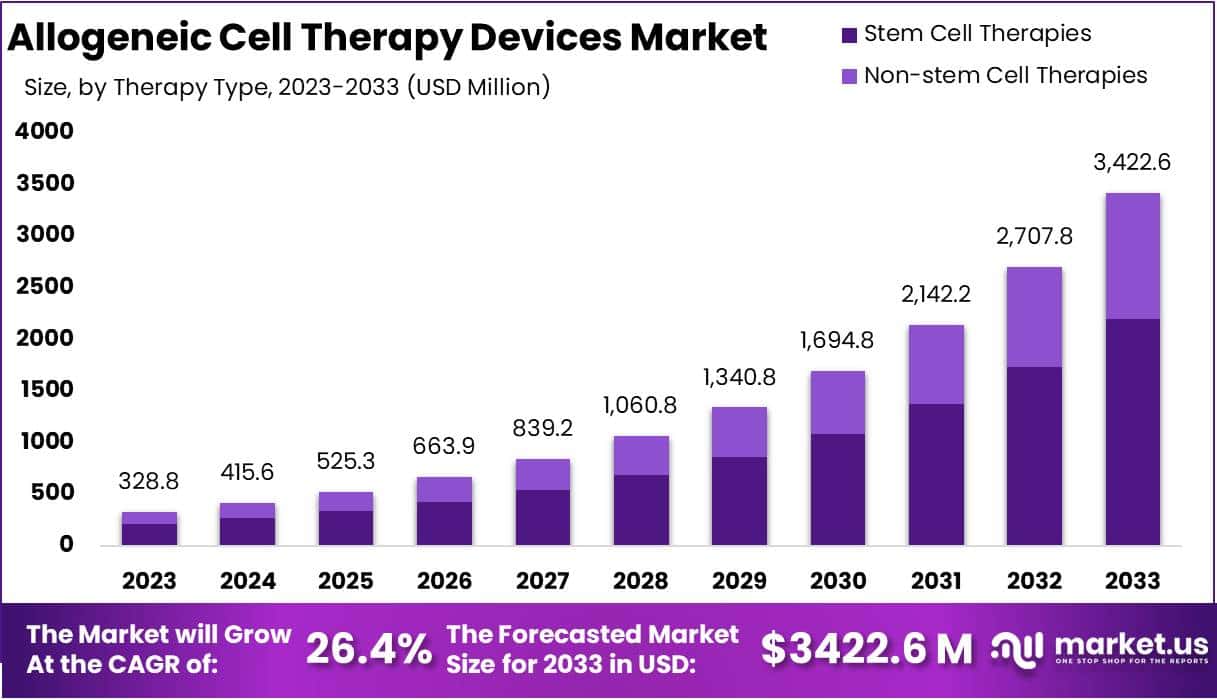
The Global Allogeneic Cell Therapy Devices Market size is expected to be worth around USD 3422.6 Million by 2033, from USD 328.8 Million in 2023, growing at a CAGR of 26.4% during the forecast period from 2024 to 2033.
The Allogeneic Cell Therapy Devices Market is a rapidly evolving segment within the field of regenerative medicine and biotechnology. It encompasses devices and technologies used in the production, processing, and administration of allogeneic cell therapies, which involve using cells from a donor for therapeutic purposes.
Key drivers of the market include the increasing prevalence of chronic diseases, rising demand for personalized and regenerative medicine, and advancements in cell therapy technologies. Allogeneic cell therapies offer potential treatments for various conditions such as cancer, autoimmune disorders, and genetic diseases. Challenges in the market include regulatory complexities, manufacturing scalability, and ensuring safety and efficacy of allogeneic cell therapies.
However, ongoing research and development efforts, along with investments in manufacturing infrastructure and quality control processes, are expected to drive market growth and enable broader adoption of allogeneic cell therapy devices in clinical practice. The market is characterized by a growing number of collaborations and partnerships between biotechnology companies, research institutions, and healthcare providers to develop and commercialize innovative cell therapy products.
- According to the Pan American Health Organization, in USA, around 4 million individuals received new cancer diagnoses, and approximately 1.4 million people succumbed to the disease in 2020. Notably, about 57% of these new cancer cases and 47% of the cancer-related deaths affected individuals aged 69 years and younger.
- As per a news release by the World Health Organization, in 2022, approximately 20 million individuals were newly diagnosed with cancer, and around 9.7 million deaths were attributed to the disease.
- A population-based study, published in the Lancet journal, involving 22 million people showed that autoimmune disorders now impact approximately one in ten individuals.
Key Takeaways
- The market for Allogeneic Cell Therapy Devices generated a revenue of USD 328.8 million, and is poised to reach USD 3422.6 million, with a CAGR of 26.4%.
- Stem cell therapy segment generated the most revenue for the market, with a market share of 64.2%.
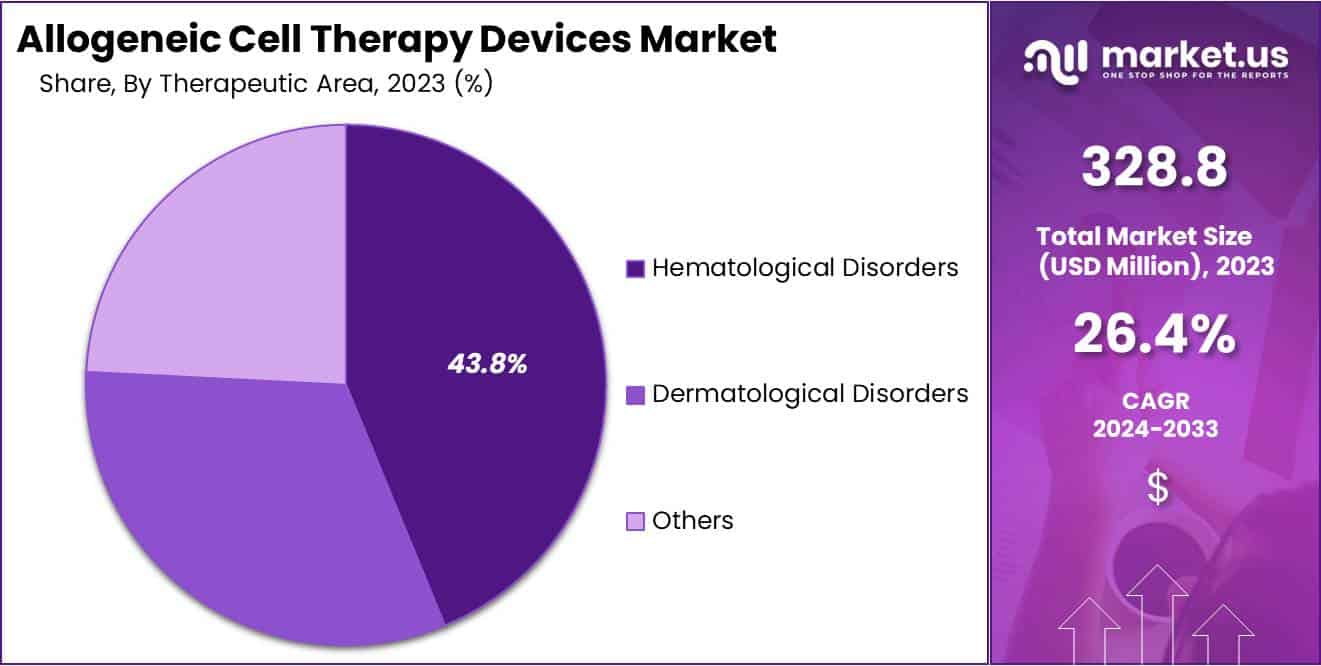
- Based on therapeutic area, the hematological disorders segment contributed the most to the market in 2023, having claimed revenue share of 43.8%.
- Among end-users, hospitals and clinics remained primary end-users in 2023.
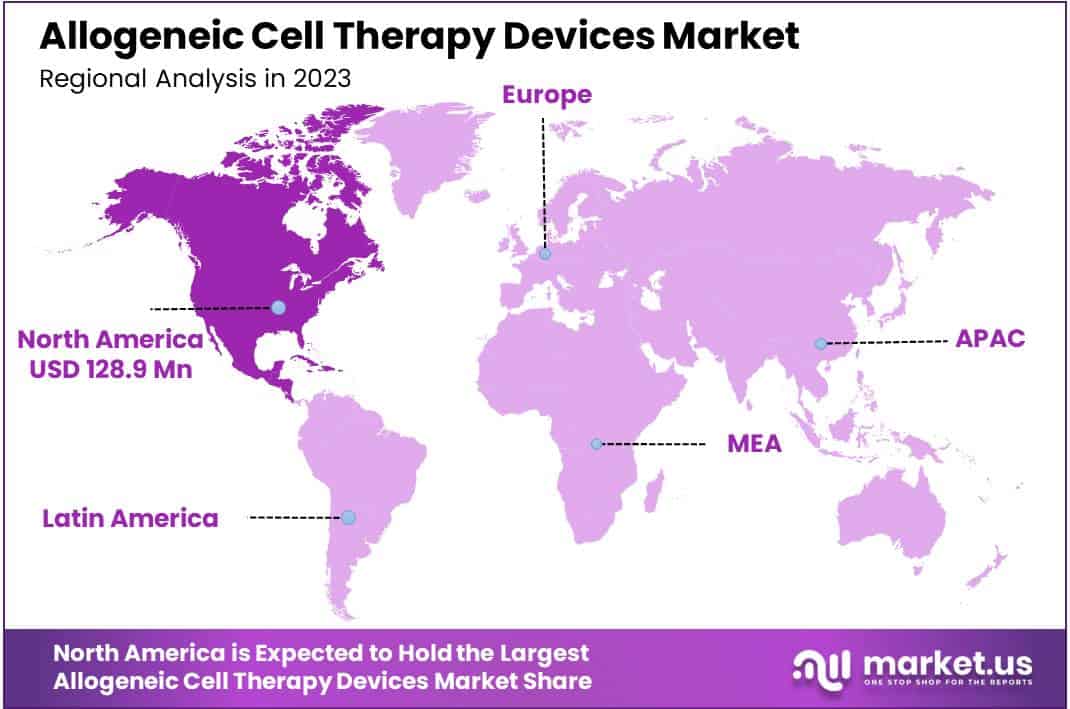
- Region wise, North America remained the lead contributor in the market with a revenue share of 39.2%.
Therapy Type Analysis
In 2023, stem cell therapy emerged as the leading revenue-generating segment in the Allogeneic Cell Therapy Devices Market with a market share of 64.2%. This therapy type harnesses the unique potential of stem cells to differentiate into various cell types, making it effective for treating a range of diseases. The dominance of stem cell therapies is attributed to the rising prevalence of conditions like leukemia, lymphoma, blood cancers, and specific autoimmune disorders, which can be effectively treated with these therapies.
On the other hand, the non-stem cell therapy segment is expected to exhibit significant growth during the forecast period. This category encompasses cell-based treatments that do not rely on stem cells but utilize differentiated cells or immune cells. Examples include T-cell therapies, dendritic cell therapies, islet cell transplantation, and chondrocyte therapies.
Therapeutic Area Analysis
In 2023, the hematological disorders segment dominated the Allogeneic Cell Therapy Devices Market, claimed revenue share of 43.8%, driven by the increasing incidence of hematological conditions globally. The growing patient population with disorders like leukemia and lymphoma contributes to this segment’s market share, especially as these diseases have a history of being treated with allogeneic cell therapies, such as hematopoietic stem cell transplantation (HSCT), which effectively replaces diseased blood cells with healthy donor cells.
Conversely, the dermatological disorders segment is forecasted to witness the fastest growth rate during the projected period. This growth is fueled by heightened awareness of allogeneic stem cell-based therapies used in treating various dermatological conditions, including wound healing, skin aging, scar therapy, atopic dermatitis, autoimmune skin disorders, among others.
End-User Analysis
The segmentation based on end users in the Allogeneic Cell Therapy Devices Market encompasses Hospitals & Clinics, Research Institutes & Academic Centers, Biotechnology & Pharmaceutical Companies, and Others. The Hospitals & Clinics segment represents healthcare providers where cell therapies are administered to patients, making it a crucial end user group for the market.
The segment claimed the highest market revenue share of 43.7%. These facilities play a central role in delivering cell-based treatments and managing patient care. The dominant segment in this segmentation is Hospitals & Clinics. They serve as the primary point of care for patients receiving cell therapies, driving the adoption and utilization of allogeneic cell therapy devices within the healthcare ecosystem.
Key Market Segments
By Therapy Type
- Stem Cell Therapies
- Non-stem Cell Therapies
By Therapeutic Area
- Hematological Disorders
- Dermatological Disorders
- Others
By End-User
- Hospitals & Clinics
- Research & Academic Institutes
- Biotechnology & Pharmaceutical Companies
- Others
Market Drivers
Increasing Prevalence of Chronic Diseases
The rising prevalence of chronic diseases worldwide is a significant driver for the Allogeneic Cell Therapy Devices Market. Chronic conditions such as cancer, autoimmune disorders, cardiovascular diseases, and neurodegenerative disorders represent a growing healthcare burden globally. According to the World Health Organization, cardiovascular diseases (CVDs) stand as the primary cause of mortality worldwide, claiming approximately 17.9 million lives annually.
Allogeneic cell therapies offer promising treatment options for these diseases, driving demand for cell therapy devices and technologies. Allogeneic cell therapies, particularly hematopoietic stem cell transplantation (HSCT) and CAR-T cell therapy, have revolutionized cancer treatment by targeting cancer cells with precision and enhancing the body’s immune response against tumors. The increasing incidence of cancer cases fuels the demand for innovative cell-based therapies. Allogeneic cell therapies hold potential for treating autoimmune diseases by modulating immune responses and restoring immune tolerance.
Growing Investment in Research and Development (R&D)
Investments in research and development (R&D) are key drivers for innovation and expansion in the Allogeneic Cell Therapy Devices Market. Pharmaceutical companies, biotechnology firms, academic institutions, and government agencies are investing significantly in R&D efforts to advance cell therapy technologies and develop novel therapies.
Collaborations between industry players, research institutions, and regulatory bodies facilitate knowledge sharing, technology transfer, and accelerated development of allogeneic cell therapies. These partnerships drive innovation and address challenges in commercializing cell-based products. Government grants, venture capital investments, and funding initiatives support R&D activities in cell therapy research. These financial resources enable companies to explore new therapeutic avenues, optimize manufacturing processes, and enhance product development capabilities.
Restraints
Limited Manufacturing Scalability
The scalability of cell therapy manufacturing presents a significant restraint for the Allogeneic Cell Therapy Devices Market. Achieving large-scale production while maintaining product quality, consistency, and safety is a challenge. Existing manufacturing facilities may have limited capacity to meet growing demand for cell therapy products.
Expanding manufacturing capacity requires substantial investments in infrastructure, technology, and workforce training. Ensuring consistent and reproducible manufacturing processes across different batches and production sites is crucial for regulatory compliance and product quality. Variability in manufacturing can impact treatment outcomes and patient safety.
High Implementation Costs
Another significant restraint for the Allogeneic Cell Therapy Devices Market is the high costs associated with cell-based therapies, including manufacturing, testing, and administration expenses. Developing and optimizing cell therapy products involves substantial investment in research, preclinical studies, clinical trials, and regulatory submissions. High R&D costs contribute to overall treatment expenses.
Cell therapy manufacturing is complex, requiring specialized facilities, equipment, and skilled personnel. The costs associated with manufacturing scalability, process optimization, and quality control can be significant. Receiving reimbursement for cell therapy treatments from payers and healthcare systems can be challenging due to uncertainties about cost-effectiveness, coverage policies, and reimbursement rates. Limited reimbursement may deter market adoption and patient access to cell-based therapies.
Opportunities
One of the main opportunities for the Allogeneic Cell Therapy Devices Market lies in the expanding applications of cell-based therapies across a wide range of medical conditions. As research and development efforts continue to advance, there is growing potential to leverage allogeneic cell therapies for treating not only hematological disorders and solid tumors but also autoimmune diseases, degenerative disorders, and genetic conditions.
Furthermore, the increasing focus on personalized medicine and regenerative therapies presents a significant opportunity for the market. Allogeneic cell therapies can be tailored to individual patient profiles, optimizing treatment outcomes and minimizing adverse effects.
Moreover, strategic collaborations, partnerships, and investments in infrastructure and technology can enhance manufacturing scalability, reduce costs, and improve accessibility to cell therapy products. These opportunities collectively contribute to the growth and expansion of the Allogeneic Cell Therapy Devices Market in the healthcare industry.
Impact of Macroeconomic / Geopolitical Factors
Inflation, interest rates, government expenditure, and policies can significantly impact the Allogeneic Cell Therapy Devices Market. High inflation rates may increase production and operating costs for manufacturers, potentially leading to higher prices for cell therapy devices. Fluctuations in interest rates can affect investment decisions and financing options for companies in the market.
Government expenditure and policies, such as funding for research and development, regulatory frameworks, and reimbursement policies, play a crucial role in shaping market dynamics, innovation, market accessibility, and affordability of cell therapy devices. Collectively, these economic factors can influence market growth, competitiveness, and investment attractiveness in the Allogeneic Cell Therapy Devices Market.
Latest Trends
The integration of CRISPR-Cas9 and other gene editing tools into cell therapy development is enhancing precision and customization of allogeneic cell therapies, leading to improved therapeutic outcomes. Also, cell-based therapies are expanding beyond traditional applications to include new therapeutic areas such as neurological disorders, musculoskeletal conditions, and metabolic diseases, broadening the market scope and potential patient population.
There is a growing emphasis on optimizing cell therapy manufacturing processes, including automation, closed systems, and scalable production platforms, to enhance efficiency, reduce costs, and meet increasing demand for cell-based treatments. Lastly, regulatory agencies are adapting guidelines and frameworks to accommodate the unique characteristics of cell therapies, streamlining approval processes, and fostering innovation in the market.
Regional Analysis
North America is leading the Allogeneic Cell Therapy Devices Market
North America’s dominance in the Allogeneic Cell Therapy Devices Market in 2023 can be attributed to several factors that contribute to its robust revenue share. Firstly, North America boasts one of the world’s most advanced healthcare infrastructures, characterized by a network of specialized hospitals, research institutions, and medical universities.
This infrastructure facilitates the development, commercialization, and adoption of cutting-edge allogeneic cell therapy devices. Additionally, the region benefits from a well-established regulatory framework, including stringent but clear guidelines for cell therapy products’ approval and commercialization. This regulatory environment instills confidence among investors, manufacturers, and healthcare providers, driving market growth and innovation.
The Asia Pacific region is expected to experience the highest CAGR during the forecast period
On the other hand, the Asia-Pacific region is poised to experience rapid growth in the Allogeneic Cell Therapy Devices Market due to substantial investments in healthcare infrastructure. Countries like China, Japan, South Korea, and India have been investing significantly in upgrading their healthcare facilities, including the establishment of specialized treatment centers and research facilities focused on cell-based therapies.
The growing healthcare expenditure, rising prevalence of chronic diseases, and increasing awareness about advanced treatment options are driving demand for allogeneic cell therapy devices in the Asia-Pacific region.
Key Regions and Countries
- North America
- US
- Canada
- Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
The Allogeneic Cell Therapy Devices Market is characterized by key players vying for market share. Major companies such as Novartis AG and Gilead Sciences, Inc. are prominent players in this market. These companies invest significantly in research and development to innovate cell therapy technologies and expand their product portfolios.
Strategic collaborations, mergers, and acquisitions are common strategies adopted by key players to strengthen their market position and gain a competitive edge. Market dynamics, regulatory landscape, and technological advancements play pivotal roles in shaping market share and the competitive landscape of the Allogeneic Cell Therapy Devices Market.
Top Key Players in Allogeneic Cell Therapy Devices Market
- Novartis AG
- Gilead Sciences, Inc.
- Celgene Corporation
- Bluebird Bio, Inc.
- Pfizer Inc.
- Allogene Therapeutics
- Other Key Players
Recent Developments
- In May 2023, Legend Biotech Corp. has initiated a collaboration with Novartis AG, specifically through its subsidiary Legend Biotech Ireland Ltd., for the exclusive global development and licensing of chimeric antigen receptor T-cell therapies focusing on DLL3.
- In July 2023, Cognizant, an IT services firm, has extended and broadened its existing partnership with Gilead Sciences, a prominent biopharmaceutical company headquartered in California. This renewed agreement is anticipated to yield a total value of $800 million over the upcoming five years.
- In January 2023, the US health regulator has mandated companies to include a boxed warning on the labels of CAR-T cancer therapies. This decision by the FDA aims to highlight the risks associated with these treatments, particularly concerning severe side effects like cytokine release syndrome and neurological toxicities.
Report Scope
Report Features Description Market Value (2023) USD 328.8 million Forecast Revenue (2033) USD 3422.6 million CAGR (2024-2033) 26.4% Base Year for Estimation 2023 Historic Period 2018-2022 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Therapy Type – Stem Cell Therapies, Non-stem Cell Therapies; By Therapeutic Area – Hematological Disorders, Dermatological Disorders, Others; By End-User – Hospitals & Clinics, Research Institutes & Academic Centers, Biotechnology & Pharmaceutical Companies, Others Regional Analysis North America – US, Canada; Europe – Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe; Asia Pacific – China, Japan, South Korea, India, New Zealand, Singapore, Thailand, Vietnam, Rest of APAC; Latin America – Brazil, Mexico, Rest of Latin America; Middle East & Africa – South Africa, Saudi Arabia, UAE, Rest of MEA Competitive Landscape Novartis AG, Gilead Sciences, Inc., Celgene Corporation, Bluebird Bio, Inc., Pfizer Inc., Allogene Therapeutics, and Other Key Players Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Allogeneic Cell Therapy Devices MarketPublished date: March 2024add_shopping_cartBuy Now get_appDownload Sample
Allogeneic Cell Therapy Devices MarketPublished date: March 2024add_shopping_cartBuy Now get_appDownload Sample -
-
- Novartis AG
- Gilead Sciences, Inc.
- Celgene Corporation
- Bluebird Bio, Inc.
- Pfizer Inc.
- Allogene Therapeutics
- Other Key Players









